Consensus Treatment Plans
Consensus Treatment Plans (CTPs) were created to help doctors study and compare common treatments to learn which ones are most effective.

What is a Consensus Treatment Plan (CTP)?
- CTPs are a specific set of treatment/intervention suggestions for providers who treat patients with rheumatic disease. The treatment/intervention options are determined by literature review combined with agreement among providers who care for children with rheumatic disease.
- CTPs were created to address situations where there is no clear best treatment choice for a particular condition.
- By narrowing treatment approaches and then studying how patients respond to the treatment choice, physicians can better identify which treatment options may be most effective.
- The ultimate aim of CTPs is to standardize care in pediatric rheumatology in order to improve patient outcomes.
CTP Fast Facts
- In 2009, the National Institutes of Health (NIH) granted CARRA funds to develop the CARRA Consensus Treatment Plan (CTP) program.
- There are currently 12 CTPs for nine different rheumatic conditions.
- CTPs give clinicians caring for children with rheumatic disease a consensus-driven treatment resource.
Which diseases have a CTP?
There are 12 CTPs for the following conditions. Click on each to read more about the CTP.
Why are CTPs important?
Rheumatic diseases in children are rare. A Consensus Treatment Plan gives doctors a resource to see which treatments are most commonly used by pediatric rheumatologists. The use of CTPs can help reduce variability in treatment.
A CTP also gives families an idea of what treatments are being used most often in other patients with the same condition.
CTPs also can be used to help researchers determine which treatments are most effective for a specific disease. Data collected from CTPs in the CARRA Registry is used for research to improve patient outcomes.
How are Consensus Treatment Plans Developed?
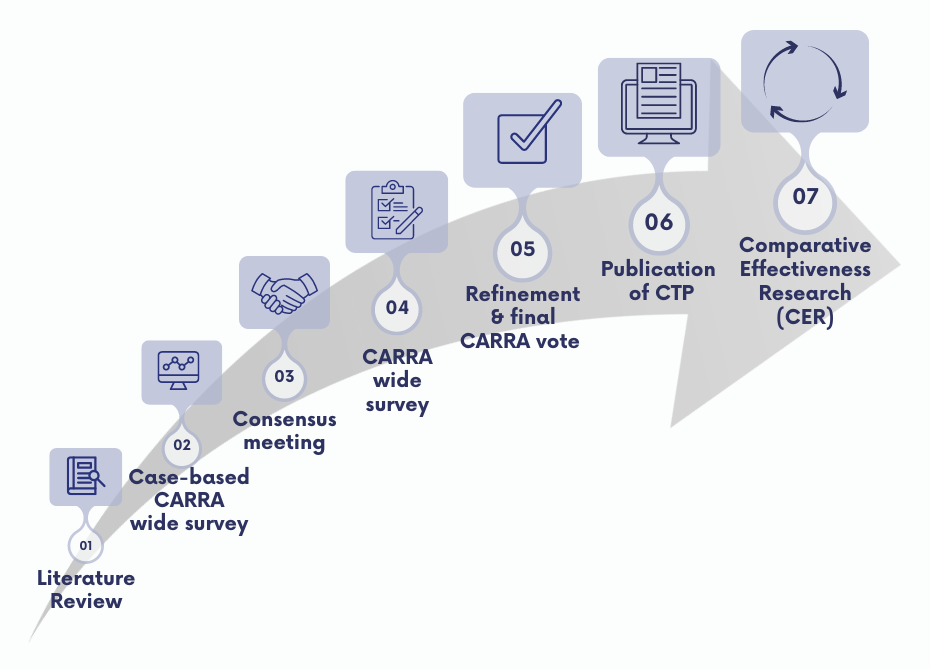
- Literature review
- Case-based CARRA wide survey
- Consensus meeting
- CARRA wide survey
- Refinement & final CARRA vote
- Publication of CTP
- Comparative Effectiveness Research (CER)
Listen to our podcast episode about the CARRA Registry

For questions, please contact [email protected].