Necessity, the Mother of Invention
How a small band of US pediatric rheumatologists found one another, found a common purpose,and founded CARRA.

Looking back no further than the 1990s, the vast unmet needs in treating oftentimes wheelchair-bound children with arthritis and other rheumatic and related autoimmune diseases then spawned a collaborative research network of pediatric rheumatologists, the Childhood Arthritis and Rheumatology Research Alliance, or CARRA.
“Early in my career, there was a family I was working with where I started their child on the standard therapeutic course at the time,” said CARRA Board member Carol Wallace, MD. “While the patient improved, we eventually decided to try a more aggressive course of treatment. The patient improved tremendously.”
Although the patient’s dramatic response didn’t surprise Dr. Wallace, very little data supported prescribing a novel, radical treatment sooner rather than later. What’s more, “the patient’s family returned, upset that I hadn’t used the more aggressive treatment sooner,” Dr. Wallace recalls. “But I didn’t have the data. I knew I needed more,” she explained.
Not until 1991 did the American Board of Pediatrics formally recognize pediatric rheumatology as a specialty, recounted two of CARRA’s founders, Yukiko Kimura, MD and Laura Schanberg, MD, in a 2016 edition of The Rheumatologist. “Prior to this time, children with rheumatic diseases were treated by a hodgepodge of providers,” that, besides trained pediatric rheumatologists, included “general pediatricians, adult rheumatologists, allergist-immunologists, orthopedists, pediatric infectious disease specialists. As a result, there was little advocacy or research in the field and little consistency in treatment approaches,” the two wrote.
Rheumatic diseases are not exactly rare.
The American College of Rheumatology estimates approximately 300,000 children in the United States living with some form of arthritis today. Pediatric rheumatologists treat myriad rheumatic disorders such as juvenile idiopathic arthritis (JIA), lupus, dermatomyositis and scleroderma.
Dr. Wallace wasn’t alone in yearning for more data to help children with pediatric rheumatic diseases – and their families.
During the CARRA Annual Scientific Meeting (ASM) in May 2022, Christy Sandborg, MD, ruefully recounted her early frustrations: “When I came to Stanford in 1997, I had done research on cytokines and cytokine inhibitors. I knew there were amazing new biologic medicines coming down the pike called TNF inhibitors that were being tested in adults. I thought these (treatments) would be transformational, perhaps in kids with arthritis.”
“In fact they were,” she continued, “which was exciting enough. But I also recognized that we had only so many pediatric rheumatologists in the nation, with fewer than 50% of these children being taken care of by pediatric rheumatologists. What’s more, only 60% of medical schools even had one pediatric rheumatologist,” Dr. Sandborg recalled. “We were graduating so few fellows a year that when I was sitting on the American Board of Pediatrics at about the same time, we heard the rumor that (the Board was) going to stop offering our boards because we only were graduating an average of seven people a year into pediatric rheumatology!”
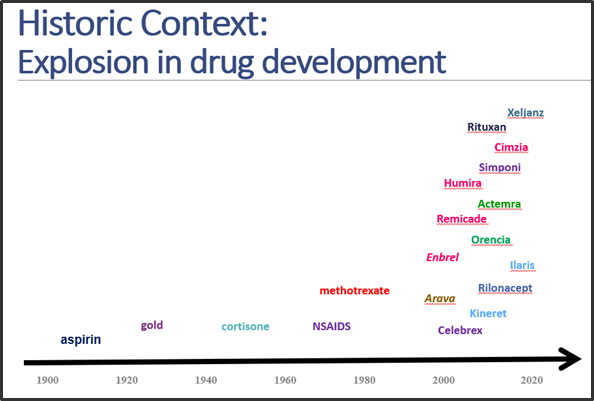
Concerns about scant data.
In the early 2000s, there were promising new biologic agents, but too few pediatric rheumatologists. There were plenty of children in the US suffering pain, disability and life-threatening conditions at worst. What’s more, a scarcity of optimal, quality data and workforce to deliver patients significant advances in treatment, never mind a cure.
Yet the wellsprings of expertise in JIA research continued to pool together, comparing notes about problems and more so the opportunities. The growing necessity brought forth invention. Via collaboration.
Dr. Sandborg began to work with her colleague Elizabeth Mellins, MD, who had come to Stanford to do research a year earlier. “Betsy was transformational in getting us into this new space,” recalled Dr. Sandborg.
“It was clear to all of us that we needed to work together,” shared Dr. Mellins at the 2022 CARRA ASM. “In fact, in order to preserve the field and move forward, we needed to formalize how we worked together. There were so few of us that we all turned to each other when we had patients who were keeping us awake at night,” she said.
Late Night Calls
“We started with weekly phone calls late at night after we’d put our kids to bed,” recalled Dr. Wallace. “We were sometimes despondent about how little we knew. But in just a couple of years, we were able to create a network to start answering those ‘burning questions.’”
“We had many research centers, but in each center there were only one or two people at most, and nobody had enough patients, so we had to combine forces,” Dr. Sandborg elaborated.
These early collaborators recognized that the pediatric rheumatology specialty lacked a representative, research-based organization to further research to help these children.
Fortunes Turn
Fortunes turned for the better in formalizing pediatric rheumatic disease research at a 1999 meeting sponsored by the Arthritis Foundation, attended by Dr. Mellins and Brian Feldman, MD, MSc. Participants marveled over a potentially transformative gift that the Minneapolis-based Wasie Foundation had granted to the Arthritis Foundation. The benefactors earmarked pediatric rheumatology as the grant’s purpose.
“Industry-led research had been incredibly important and often aligned with our goals,” said Dr. Feldman. He proposed a researcher-led network that would push the field further in the right directions.
“I was really taken with Brian Feldman’s idea that the best way to apply the funding was to try to actually formalize the way we were working together and to try to get to come together as a research network, particularly focused on investigator-initiated research, since we all had questions,” said Dr. Mellins.
“What we lacked were the mechanisms to work together to answer these questions,” continued Dr. Mellins.
Both Drs. Yukiko Kimura and Laura Schanberg were invited to one of those first meetings and met for the first time. Dr. Kimura recalled: “We both felt more than a little intimidated, yet these thought leaders made sure that we knew our voices were just as important as theirs.”
Catching Fire
The idea to formalize caught fire. In Fall 2000, Dr. Mellins and Dr. Sandborg held a conference in Palo Alto to propose the network.
Dr. Mellins recalled, “When we started looking around at other networks and how they functioned, it became clear they were also training grounds for people and that they were an infrastructure that allowed people to learn how to do research, which also gave them the tools to do research, which we wanted to emulate as we designed our network.”
“We learned about other networks and decided with Carol Wallace, Dr. Norm Ilowite and some others to take the next step. At our next meeting, held in Chicago, with Dr. Ed Giannini cleverly coming up with some money for us to meet, we had about 25 to 30 pediatric rheumatologists there,” Dr. Mellins recalled. Among the participants of this meeting were all the future Chairs/Presidents of CARRA: Aside from Christy Sandborg and Betsy Mellins, Carol Wallace, Norman Ilowite, Laura Schanberg, Yukiko Kimura, Rob Fuhlbrigge and Emily von Scheven all participated in the founding of the organization.

This meeting gathered CARRA’s founding pediatric rheumatologists, representatives from the NIH, the Arthritis Foundation, and the Wasie Foundation to codify a collaborative, cooperative network of pediatric rheumatologists and investigators in the U.S. and Canada. These discussions, supported by the American College of Rheumatology and grants from the Wasie Foundation, the Lucile Packard Foundation, and the Arthritis Foundation again, evolved to a grassroots initiative to create a research network. The new network would support engagement of all providers, researchers and patients, develop standards of care, and promote the development of a culture of research within the community of pediatric rheumatology providers. One important activity resulted in a set of guiding principles that still apply today, through a process led by Dr. Balu Athreya.
“One of the biggest principles was that every pediatric rheumatologist here in academic centers could actually participate in the research,” added Dr. Sandborg.
In naming the new organization, the founders chose “Childhood Arthritis and Rheumatology Research Alliance” or CARRA, reminiscent of the Latin cura, or “care.”
The word “care” applied to patients and researchers alike. “How to develop research together and truly collaborate,” notes Dr. Kimura, “is also what CARRA stands for.”
After building a foundation over two years, the organizers launched CARRA in 2002 by ratifying by-laws and conducting a membership drive.
Business and legal advisor Leigh White, JD, (also a parent of a pediatric rheumatology patient) helped to develop and initially led the governance structure. “It was impressive how quickly our field had been withering,” she said. “Our goal in governance was different than people might initially think. We wanted to educate investigators and encourage people to go into the field.”
This structure also ensured researcher access to as much patient data as possible.
Most of the funding for early research studies and clinical trials came from NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases), the Arthritis Foundation, Lupus Foundation of America (LFA), Cure Juvenile Myositis (CureJM), the American College of Rheumatology and Friends of CARRA, with the Duke Clinical Research Institute (DCRI) functioning as the data coordinating center for CARRA’s multi-center clinical trials.
Patient Involvement
A more concerted effort to involve patients, and their families, equipped CARRA with a 360° view of pediatric rheumatic and other related diseases, their treatments, and their real-life implications.
“The diseases we treat are chronic. Patients and families have to be part of the treatment plan, and pediatric rheumatology has been ahead of the curve when it comes to engagement,” Dr. Feldman explained. “A parent named Vincent Del Gaizo understood that CARRA should partner with patients and families.”
Vincent’s son developed JIA and required intensive treatment. Vincent and his family began working with Dr. Kimura on a plan for his son’s care. “Being involved in my son’s treatment and contributing any way I could became my life’s work,” Vincent shared. “Patients and families can help guide research to answer the critical questions in pediatric rheumatology.”
Vincent demonstrated a talent for developing patient-family partnerships within CARRA regardless of a family’s background. “Every lived experience is real and means something to the research,” Vincent said. “Experience can answer a question, or open a new question. The research doesn’t move forward without a diversity of experience,” he shared.
Dr. Kimura agrees that it was Vincent’s son who drove home to her how vital patient partnerships are in pediatric rheumatology. “We not only need to collaborate with each other as researchers, but we also need to collaborate in just the same way with our patients. Our patients’ questions are the genesis of much of our research today,” she added.
CARRA’s first research grant was the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) Trial. Written by Drs. Sandborg and Schanberg, and awarded by the NIH in 2002, the grant funded a seminal study of pediatric lupus that involved 22 sites in the U.S. and Canada and enrolled the largest ever prospective cohort of these patients.
In particular, APPLE studied the impact of atorvastatin on children with lupus, who experience higher rates of heart disease resulting not only from the disease but their treatments.
“It was a very complex study to perform,” Dr. Sandborg related. “It showed, though, that we had the ability to do research beyond what had been done in adult lupus research.”
CARRA has grown from an organization of 20 to more than 550 pediatric rheumatology researchers in 2022. Thanks to the dedication of CARRA’s investigators, research coordinators, patients, families and staff, and the support of funders, more than 20,000 patients have participated in CARRA clinical trials, registries and other CARRA research studies.
“The future of academic pediatric rheumatology seems brighter than ever, with a growing clinical and research workforce and improving research infrastructure, as well as an array of effective medications to treat pediatric rheumatic diseases and prevent life-altering disability,” wrote Rob Fuhlbrigge, MD, PhD, along with Dr. Kimura and Dr. Schanberg, in Rheumatic Disease Clinics, September 2021.
“However, there is still much to do.”

Necessity, the mother of invention, powering CARRA’s future.
Sources:
- The Future of Pediatric Rheumatology Grounded in Evolution of Childhood Arthritis and Rheumatology Research Alliance, The Rheumatologist, December 15, 2016; Yukiko Kimura, MD; Laura E. Schanberg,MD
- Rheum Dis Clin N Am VOLUME 47, ISSUE 4, P531-543, NOVEMBER 01, 2021; Robert C. Fuhlbrigge, MD, PhD; Laura E. Schanberg, MD; Yukiko Kimura, MD
Acknowledgments:
Appreciation to Stacy Ardoin, MD; Kelly Mieszkalski; Leslie Hanrahan; Karin Tse; Jen Kowalski; Victoria Nelson and Larry Hausner
Our Growth
Our founding members quickly realized that one of the best ways to achieve its primary focus was to begin a registry project. The CARRA Registry was founded as a means to collect information about how childhood-onset rheumatic diseases are treated and how they affect patients. The information is provided by the medical team and by patients and their families. Patients’ names and other identifying information are kept separate from all of the other information.
The registry was created to monitor the long term safety of the medications used to treat pediatric rheumatic diseases. A recent survey of parents listed understanding long term safety of medications as their #1 priority. But the registry does so much more: It also collects large amounts of information on thousands of patients, their treatments, side effects and quality of life, so you will be able to make more informed decisions about your/your child’s health. The goal is to have all the information you need to choose the safest treatment for your desired outcome.
Researchers use the information collected to answer important questions about how safe medications are, how well medications work, and how well patients do over time. Combining the Registry information with biosamples, such as blood tests or genetic tests, can be a very powerful tool for researchers. For example, they may be able to predict how well patients will do with a particular medication or even find a cure for the disease.
CARRA members work together to capitalize on opportunities, actively develop a structure and a scientific agenda, and collaborate with potential funding sources to create a research alliance that benefits our patients.
Our Patients
CARRA believes that patients who are affected by pediatric rheumatic diseases and their families should help us lead research efforts in these areas. That means being part of the research team. Team members help define the questions that need to be answered, design research studies and protocols, develop patient/public facing materials, and translate medical articles to make them understandable to patients and families.
Our Values
CARRA wants remain true to our founding principles and achieve our ever-expanding goals. With that in mind, we strive to support studies that can help build an evidence base for effective care for children with rheumatic disease. We want to give priority to investigator-initiated protocols, and assign priority for research development on the basis of scientific merit and impact. We encourage our research sites and members to commit to enrolling patients in research studies. We want to institute transparent and clearly articulated operating principles, including organizational structure, governance, protocol selection, protocol development, and publication mechanisms.
Internally, we elect alliance leadership in a democratic fashion. We ensure high standards of performance, accountability, and ethics for members. Developing and providing resources to centers and investigators to maximize participation in research studies is of great importance. We also encourage and support inclusion of epidemiologic and translational research in association with clinical studies, and embrace data sharing and dissemination of results as a core principle.
Looking towards the future, we are committed to developing protocols for all rheumatic diseases of childhood, and work to respond rapidly to funding opportunities. Lastly, we seek to foster relationships with members of other organizations who support CARRA’s mission and vision.
Our Goals
Since CARRA’s inception, we have published numerous scientific articles, funded various grants, and launched major studies. We also host an annual scientific meeting. Our goal is to enroll 10,000 patients in our registry – and we have achieved that goal!
CARRA’s Historical Leadership
- 2002-2004: Elizabeth (Betsy) Mellins, MD, Chair
- 2004-2007: Christy Sandborg, MD, Chair
- 2007-2010: Carol Wallace, MD, Chair
- 2010-2013: Norm Ilowite, MD, Chair
- 2013-2016: Laura Schanberg, MD, Chair and CARRA’s 1st President*
- 2016-2018: Yukiko Kimura, MD, President
- 2018-2020: Robert Fuhlbrigge, MD, PhD, President
- 2020-2022: Emily Von Scheven, MD, MAS, President
- 2022-2024: Robert Colbert, MD, PhD, President
*CARRA incorporated as a 501(c) 3 nonprofit organization in 2014. Prior to incorporation, CARRA leaders were “Chairs” of CARRA.